
Often employing several expansion and compression steps. Liquid Gas Production - The Liquefaction of Gases such as Ammonia, Chlorine, Nitrogen, Oxygen and Argon is achieved through expansion of the pressurised gases.This is often employed in LPG production, with cooling the first step before fractionation is used to refine the liquids further. Gas separations - By cooling a gas through an expansion the heavier components will condense into a liquid phase.Air conditioning - Identical to a refrigerator in principal, but optimised for a building, vehicle or other inhabitable space.Refrigeration - The pressurisation and expansion of refrigerants in a closed loop systems are the basis of most refrigerators and freezers.Some applications where the cooling of expanding gases is employed are: Above this temperature the gas heats on expansion, below this temperature the gas cools on expansion.Īpplications of the Joule-Thomson effect generally use the cooling potential, rather than heating, as there are almost always more convenient ways to heat a process. The inversion temperature is the temperature of a gas at which a reduction in pressure causes no temperature change.
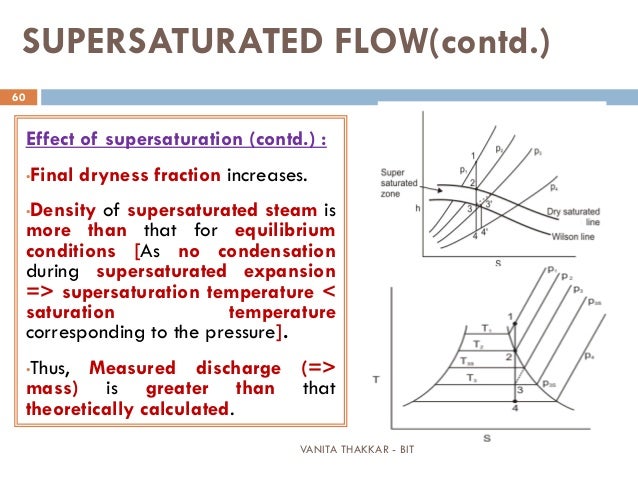
Therefore the direction of the temperature change is dependent on the upstream temperature and pressure of the gas relative to its inversion temperature. For any gas the temperature could either increase or decrease depending on how the internal energy has to change to keep enthalpy constant. Depressureing is an isenthalpic process which means enthalpy remains constant. As kinetic energy is realised as temperature this means that a reduction in temperature will be observed.Īlthough it is often generalised that for most real gases there is a decrease in temperature during a pressure reduction this is not true for all gases and conditions. Given that the potential energy component will increase as the pressure of the gas is lowered the kinetic energy component of internal energy must decrease. This implies that internal energy must decrease as the gas passes through the restriction.


 0 kommentar(er)
0 kommentar(er)
